Views: 0 Author: Site Editor Publish Time: 2025-08-26 Origin: Site








PET does not break down easily in most situations. But it can still get weaker over time, especially if it is hot or wet. It is important for makers and users to know about this resistance because:
They add things like anti-hydrolysis agents and epoxy chain extenders to keep PET safe from water damage.
These things help PET last longer and work better, even after recycling.
Better resistance helps the plastics industry be more eco-friendly by letting PET be used in tough and green ways.
How stable PET is depends on its structure and heat. For example, crystalline PET does not break down by enzymes. Amorphous PET is easier to break down, especially when it gets hotter than its glass transition temperature.
PET does not break down easily from water. Its chains are packed close together. It has special rings that stop water and enzymes from breaking it.
Heat, wetness, and strong chemicals make PET break down faster. This makes PET weaker and less bendy as time goes on.
Using anti-hydrolysis agents helps PET stay strong. Drying PET before using it also helps it last longer.
Some PET types can handle heat and wetness better. These work well in cars and electronics.
Hydrolysis helps recycle PET by breaking it into smaller parts. These parts can be used again. This helps cut down on waste and keeps the environment safe.
Hydrolysis is when water breaks down big molecules called polymers. Water gets into the polymer chain and splits the bonds. This makes the long chains in plastics like PET turn into smaller pieces or their building blocks. Hydrolysis can make plastics weaker, so they do not last as long. It happens faster if the plastic gets hot, is under pressure, or touches some chemicals. Scientists use special tools to watch how hydrolysis changes plastics. These tools show how the chains break and how the plastic loses strength and bends less.
Note: Hydrolysis can make plastics crack, lose their shape, and even fall apart if it goes too far.
PET is special because it does not break down from hydrolysis easily. Its chemical structure helps it stay strong. PET has a semi-crystalline structure, so some parts have chains packed close together. These packed parts stop water and enzymes from getting in and breaking the bonds. The tight packing also keeps the chains from moving, which helps PET stay tough.
The ester bonds in PET are inside a hard, stable area. This makes it harder for water to reach and break them. PET also has aromatic rings, which are groups of atoms that make it even stronger. These rings make PET more hydrophobic, so water cannot get in easily. If scientists change the aromatic rings or add things that like water, PET gets weaker and breaks down faster.
PET stays strong because:
High crystallinity blocks water and enzymes.
Rigid chain packing keeps the structure tough.
Aromatic rings stop water from getting in.
Plastics with less crystallinity or looser chains break down faster. PET’s special structure helps it stay strong and useful, even in hard situations.
Hydrolysis changes how PET acts. When PET meets water and heat, its long chains break into smaller ones. This makes the material weaker and lowers its molecular weight. PET starts out strong and bends easily. After hydrolysis, it gets stiff and breaks more easily. The surface can get cracks, lines, and turn yellow. Scientists use special microscopes to see these changes. They find that PET fibers get hurt, especially after soaking in strong chemicals like alkali.
PET breaks in a different way after hydrolysis. It bends less and snaps faster.
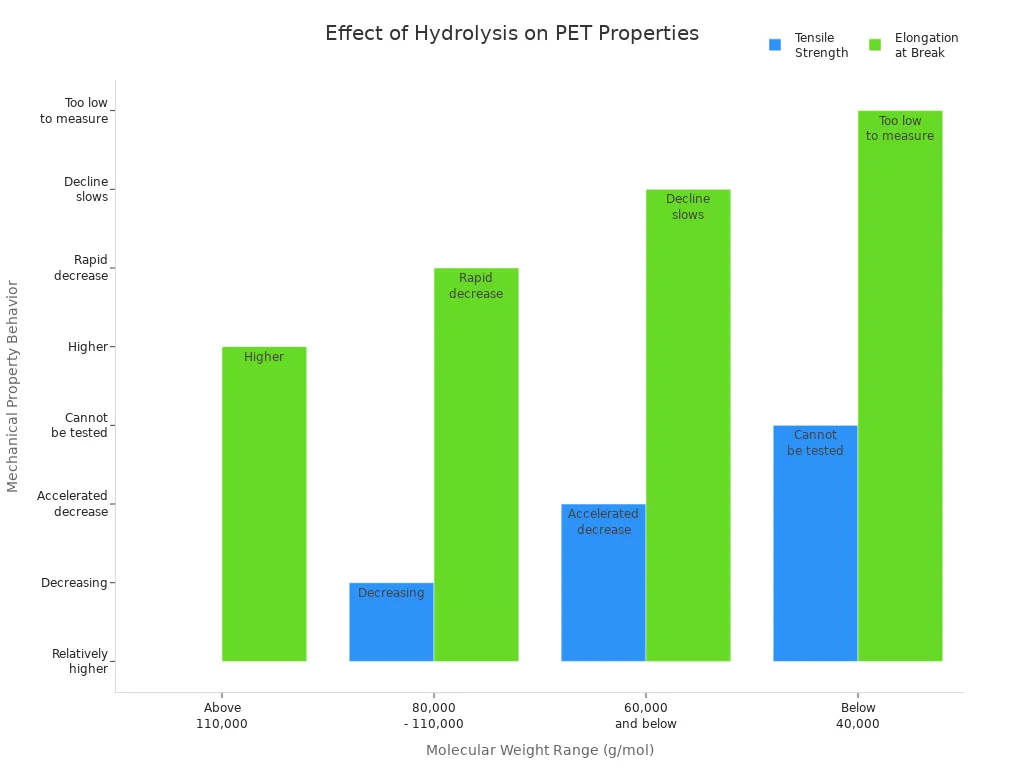
Hydrolysis also makes more crystals form in PET. When the chains break, new crystals grow inside the plastic. This makes PET harder but not as stretchy. The table below shows how PET’s strength and stretch change with molecular weight:
Molecular Weight Range (g/mol) | Tensile Strength Behavior | Elongation at Break Behavior | Additional Notes |
|---|---|---|---|
Above 110,000 | Relatively higher tensile strength | Higher elongation at break | Material remains ductile |
80,000 - 110,000 | Decreasing tensile strength | Rapid decrease in elongation at break | Embrittlement begins |
60,000 and below | Accelerated decrease in tensile strength | Elongation at break decline slows at lower MW | Material becomes brittle |
Below 40,000 | Tensile bars cannot be prepared or tested | Elongation at break too low to measure | Material too brittle for testing |
Hydrolysis makes PET products not last as long. As PET gets weaker and more brittle, it cannot handle stress or bending. Bottles, fibers, and films may crack or break sooner than you think. High heat and wetness make this happen faster. PET that goes through hydrolysis often turns cloudy and yellow. This shows it is getting damaged. The surface can wear away and crack, making it unsafe to use.
Tip: PET lasts longer if kept dry and cool. Makers use additives and special types to slow hydrolysis and help PET last longer.
PET’s resistance to hydrolysis helps it stay useful for years. But when it faces tough conditions, it gets weaker and does not last as long. People who use or make PET should watch for signs of hydrolysis to keep PET safe and strong.
Temperature and moisture are important for PET. When it gets hotter, PET molecules move more. This lets water get in and break the chains. PET starts to change at about 250°C. As it gets hotter, the reaction goes faster. This follows something called Arrhenius kinetics. That means heat makes the reaction much quicker. The energy needed for this is about 135 kJ/mol. This is important when recycling or using PET in hot, wet places.
Moisture also makes hydrolysis go faster. More water in the air means PET breaks down quicker. How fast PET breaks depends on how much water touches it. If the air is very humid, the reaction speeds up a lot. Even small changes in moisture can make a big difference.
Aspect | Effect on PET Hydrolysis |
|---|---|
High Temperature | Increases reaction rate and chain mobility |
High Humidity | Speeds up hydrolysis, especially at the surface |
Water Diffusion | Higher at warm temperatures, helps water reach inside chains |
Tip: Keep PET cool and dry to help it last longer.
Some chemicals make PET break down faster. Strong acids and bases help water attack PET bonds. This makes PET fall apart more quickly. Some solid acid catalysts can also speed up this process. Enzymes like cutinases and PETase work in mild conditions. But they need the right temperature and PET type to work well. Acidic or basic places make PET bonds easier to break. This is why PET should not touch harsh chemicals if you want it to last.
How PET is built changes how it resists hydrolysis. PET with high crystallinity has tightly packed chains. This keeps water and enzymes out. Amorphous PET has loose chains and breaks down faster. Water can reach the bonds more easily in this type. Some special enzymes can break down semi-crystalline PET. But most enzymes work better on less ordered PET. The length of PET chains matters too. Shorter chains make PET brittle and weak. PET with longer chains and more crystals stays strong for a longer time.
Factories add special chemicals to help PET last longer. These are called anti-hydrolysis agents and stabilizers. They stop water from breaking the plastic chains. Carbodiimide-based additives, like CDI and PCDI, work really well. They fix broken ends in the polymer and make it strong again. The table below shows how each additive helps:
Additive Type | Example(s) | Effect on Hydrolysis Resistance | Notes |
|---|---|---|---|
Carbodiimide-based | CDI, PCDI, HyMax® 1010 | Greatly improves resistance by fixing broken ends in PET chains | Works best at ≥1.0% concentration |
Divalent cations | Ca2+ | Helps enzymes break down PET, not for direct stabilization | Used in special recycling processes |
Surfactants | SDS | Helps enzymes, not for direct PET stabilization | Used in lab settings |
Carbodiimide additives help PET keep its strength and color. They also help PET keep its shape in wet places. These agents make a shield around the polymer. This stops water from hurting the plastic. Because of this, PET products last longer and stay strong.
Drying PET before making things is very important. PET takes in water from the air. If it is not dry, water can break the chains when melted. This makes the plastic weak and easy to break. To stop this, factories dry PET at 65-75°C for 6-8 hours. The moisture must be less than 0.005%. Some use higher heat, like 150-170°C, for 4-6 hours. Good drying keeps PET strong and stops problems. It also helps the plastic melt and flow better.
Tip: Always check drying time, temperature, and airflow. This keeps PET safe from hydrolysis.
Some PET types are made to handle more heat and water. These special grades use materials that stop water and heat from breaking the chains. For example, Lumirror™ films last longer in steam and hot places. They keep their strength and shape, even at 140°C. Many car and electronics parts use these grades. They need to work in tough places. The table below shows where they are used:
Application Area | Examples | Why Use Heat-Resistant PET? |
|---|---|---|
Automotive | Sensors, connectors, lighting | Stays strong in heat and humidity |
Electronics | Circuit breakers, plug-in connectors | Keeps shape and strength at high temps |
Housing | Power steering covers, actuators | Lasts longer in wet, hot places |
Heat-resistant PET grades help products last longer and work better in hard places.
Chemical recycling uses hydrolysis to break PET into its basic parts. This helps get important materials back for new things. There are many ways to do this, and each has good and bad points:
Hydrolysis breaks PET into terephthalic acid and ethylene glycol. This step is needed to turn old plastic into new things.
Acid hydrolysis works quickly but makes lots of waste and can hurt machines.
Alkaline hydrolysis gives pure terephthalic acid but needs special waste care.
Neutral hydrolysis does not make waste but uses lots of energy and high pressure.
Glycolysis uses ethylene glycol to break PET at lower heat. This way is used a lot in factories.
Methanolysis uses methanol and is good for nonstop recycling but needs hard steps to separate things.
New ways, like microwave-assisted hydrolysis, make the process faster and save energy.
Enzyme-catalyzed recycling uses special proteins to break PET. This way is gentle on nature but takes a long time.
The table below shows how these ways compare:
Method | Process Description | Advantages | Disadvantages/Limitations |
|---|---|---|---|
Acid Hydrolysis | PET reacts with strong acids at 200–250°C producing terephthalic acid (TPA) and EG | Fast PET breakdown | High wastewater, salt pollution, acid corrosion |
Alkaline Hydrolysis | PET reacts with strong bases (e.g., NaOH) at 60–120°C producing pure TPA | Produces pure TPA | Base waste treatment challenges, corrosion costs |
Neutral Hydrolysis | PET reacts with high-pressure water (200–260°C, 1.5–2.1 MPa) producing TPA and EG | No waste byproducts | Energy-intensive, low-purity TPA requiring refinement |
Glycolysis (Alcoholysis) | PET reacts with ethylene glycol at mild conditions producing BHET | Mild conditions, lower catalyst cost, commercial use | Requires catalysts for efficiency |
Methanolysis | PET reacts with methanol producing dimethyl terephthalate (DMT) and EG | Suitable for continuous production | High costs due to complex separation |
Ionic Liquid Catalysis | Uses ionic liquids as catalysts for mild, fast reactions producing BHET | Green, efficient, high conversion | High cost, scalability issues |
Microwave-Assisted | Uses microwaves to speed up depolymerization | Energy saving, faster reactions | Still under research |
Enzyme-Catalyzed | Uses enzymes from bacteria/fungi to degrade PET | Environmentally friendly | Low production, slow reaction rates |
Many new recycling ways use less energy and make less waste. These new ideas help make recycling safer and better.
Recycling PET with hydrolysis helps cut down plastic trash and saves resources. Chemical recycling turns old plastic into new things, so we need less oil. Some ways, like neutral hydrolysis, do not make bad leftovers. Others, like acid or alkaline hydrolysis, can pollute if not handled right.
Factories must be careful with waste and energy. High heat and strong chemicals can hurt nature. New ways, like microwave-assisted hydrolysis and enzyme-catalyzed recycling, use less energy and make less pollution. These ways help keep the earth clean and safe.
Recycling PET with better ways means less pollution and more good products. Picking the best way helps the planet a lot.
PET is good at resisting hydrolysis. But it can still break down if it gets hot or wet, or if certain enzymes touch it. Makers can help protect PET by adding anti-hydrolysis agents. They also control how much water and heat the PET gets. They try to keep the surface area small. People using PET should keep it away from high heat and lots of moisture. They should pick products with special additives. PET is weakest when it meets biological enzymes or tough places.
Using the right additives and careful steps helps PET last longer and stay safe.
PET has a strong structure. Its chains are packed tightly. PET chains have aromatic rings. These features block water and enzymes. Most other plastics do not protect as well.
PET can handle some heat and moisture. High temperatures and humidity can still weaken PET. Using special additives helps PET last longer. Heat-resistant grades also help in tough places.
Manufacturers add anti-hydrolysis agents and stabilizers. They dry PET before making products. These steps help PET keep its strength and shape. PET stays strong even with water or heat.
Hydrolysis breaks PET into smaller parts. This helps chemical recycling work. Factories use hydrolysis to get raw materials from old PET. Good recycling methods reduce waste and save resources.